This is an archival copy of the Visualization Group's web page 1998 to 2017. For current information, please vist our group's new web page.
Molecular dynamics of Cs-montmorillonite
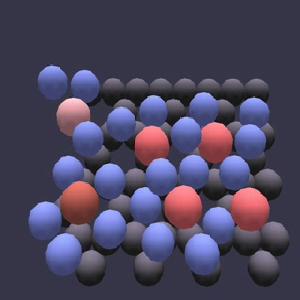 |
Montmorillonite is a common clay mineral with a layered structure and a range of permanent negative charge which allows cations and water molecules to enter the space between clay layers, also known as the interlayer. Nuclear waste containment facilities feature liners made primarily of montmorillonite, because it is thought that this clay mineral will impede the diffusion of radioactive cations such as 134Cs+ into the outer environment. Click on the image to see an animation of a molecular dynamics simulation of the interlayer region of Cs-montmorillonite. The top clay layer has been rendered invisible to allow us to see the motions of the interlayer Cs+ and water molecules, while gray spheres represent the surface plane of oxygen atoms from the bottom clay layer. Water molecules are represented by blue spheres, and Cs+ are represented by spheres of warmer colors. In this particular system, we are watching the act of cation exchange, in which one cation leaves a position near a negative charge site within the clay structure, and another cation moves to take its place. The cations performing the exchange reaction are colored red and brown, respectively, although they turn pale to signal when they are near the clay charge site.
For more information on this simulation, please see: Sutton R. and Sposito G. (2001) Molecular simulation of interlayer structure and dynamics of 12.4 Å Cs-smectite hydrates. Journal of Colloid and Interface Science 237, 174-184.
[an error occurred while processing this directive]